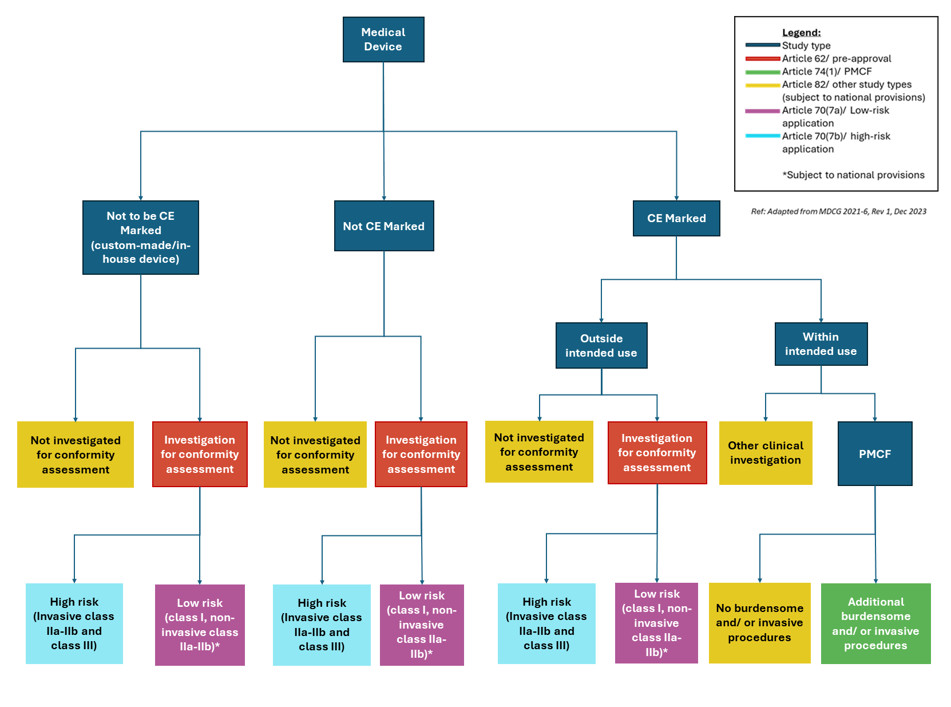
This is a comprehensive review of the process, pitfalls, and procedures to get study approval for medical device clinical trials. We look into the future and unpack the present, in terms of European medical device regulation and the local regulatory landscape. Along with the intricacies of EU MDR 2017/745 medical device regulations, we provide an important summary of medical device study classifications and the corresponding submission pathways.
Read MoreNews
Transitioning studies approved under the Clinical Trials Directive 2001/20/EC (CTD) to the new Clinical Trials Regulation 536/2014 (CTR) is a common challenge for sponsors. This blog will provide an overview of how to transition a study to the CTR and some key considerations.
Read MoreRecently, it was the 10-year anniversary of the founding of Lumis International. Here the founders reflect on the lessons learned during that time.
Read MoreWe sat down with Heike Schön our Managing Director to discuss how our clients can remain inspection ready this year, Clinical Trials Information System (CTIS) and to find out what Lumis have in store for 2023!
Read More