Selecting an authorised representative (EC-REP) is a critical step to entering the market for non-EU medical device manufacturers.
Although many companies consider this a compliance “tick box” exercise, a proactive approach can transform this relationship into a powerfully strategic one.
This article highlights how to leverage your EC-REP for valuable insights, improved efficiency, and to gain a competitive advantage.
What is an EC-REP?
First, let’s clarify what an authorised representative/ EC-REP is. According to Article 11 (1) of the EU Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), any manufacturer located outside the EU must appoint an EC-REP before placing their device(s) on the market.
The responsibilities of the EC-REP are listed in Article 11, some of the activities include:
- Verifying the device’s documentation and Declaration of Conformity exist and have undergone the correct conformity assessment
- Storing technical documentation to provide to competent authorities, upon request
- Registration – ensure the manufacturer and device are registered in EUDAMED and any other local databases
- Acting as the liaison between the competent authorities and manufacturer
- Vigilance activities – support the manufacturer with handling vigilance reports and corrective and preventative actions
Unlocking Strategic Value:
While fulfilling these core responsibilities is essential, a forward-thinking EC-REP can offer much more. Here’s how to transform your EC-REP into a strategic partner:
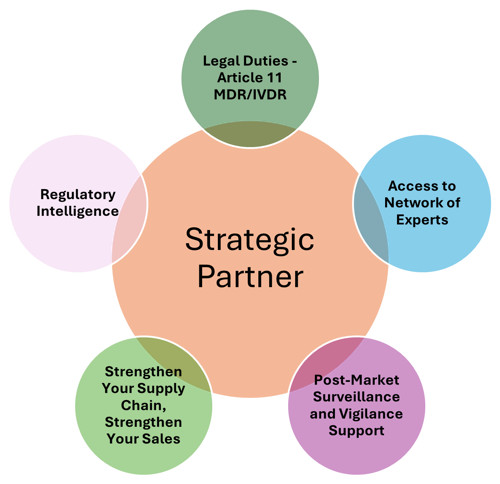
- Regulatory Intelligence: A well-connected EC-REP shares valuable insights into the evolving regulatory landscape, including upcoming changes, interpretation of existing regulations, and market trends. They provide proactive guidance, helping you anticipate challenges and capitalize on opportunities. Ask your EC-REP about:
- Upcoming regulatory changes and their potential impact on your devices.
- Strengthen Your Supply Chain, Strengthen Your Sales: Your locally-based authorised representative can provide access to a range of distributors and importers in their network. This opens a broader supply chain, enhancing market penetration and sales.
- Post-Market Surveillance and Vigilance Support: Post-market surveillance is a crucial aspect of regulatory compliance. A proactive EC-REP assists with:
- Supporting vigilance reporting and incident investigations.
- Advising on corrective and preventative actions
The authorised representative will also be a focal point for product feedback which can be used to improve product performance, safety, and customer satisfaction.
- Access to a Network of Experts: Experienced EC-REPs offer broad regulatory consulting services and often have links to additional experts like legal specialists, local marketing and sales teams, contract research organisations, and others. An established authorised representative should be your strategic coordinator in Europe.
Choosing the Right EC-REP:
Selecting the right EC-REP is crucial for maximizing the strategic value of this relationship. Consider the following criteria:
- Expertise: Look for an EC-REP with a proven track record in medical device regulations and in-depth knowledge of the MDR/IVDR. Be sure they understand their responsibilities in detail and have solid procedures in place to manage their regulatory obligations.
- Communication and Responsiveness: Choose a representative who is readily accessible, communicates clearly, and responds promptly to inquiries.
- Network and Resources: Evaluate their network of contacts and resources, including the ability to provide local support and access to specialized expertise.
- Proactive Approach: Seek an EC-REP who demonstrates a proactive approach to compliance and offers strategic guidance beyond basic requirements.
Conclusion:
By viewing your EC-REP as more than just a compliance requirement, you unlock significant strategic value. A well-networked and experienced representative provides valuable market intelligence, streamlines communication, and supports post-market surveillance, while providing access to a range of experts.
Choosing the right authorised representative and fostering a strong partnership ensures a significant advantage for medical device companies navigating the complexities of EU regulations and achieving long-term commercial success.
How can Lumis Help?
Lumis’ authorised representative service uniquely combines medical device regulatory expertise and a vast network to provide a strategically powerful advantage to our clients. We have decades of experience in medical device regulations and we specialise in representing clients in Europe.
Lumis’ state-of-the-art procedures ensure we comply with our EC-REP legal obligations, but we also offer a range of services and work with carefully selected partners to provide an all-encompassing solution.
If you want to bring your medical device to Europe, contact us for more information.

