Are you a non-EU medical device manufacturer? If so, Lumis could be your gateway into Europe via our new authorised representative service.
In accordance with Article 11 (1) of the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), any manufacturer not established within an EU Member State must designate an Authorized Representative to market their devices within the EU. This service is crucial for ensuring compliance with EU regulations, especially given the complexity and stringent requirements of the MDR and IVDR.

Comprehensive Support for Global Compliance
Lumis, headquartered in Germany, is strategically positioned to offer Authorized Representation Services that cater to the diverse needs of international medical device manufacturers. Our services are designed to facilitate smooth market entry into the EU by ensuring that all regulatory, quality, legal, and technical documentation complies with the MDR and IVDR standards. We have established state-of-the art procedures to ensure compliant but rapid access to the European market – here’s how we do it:
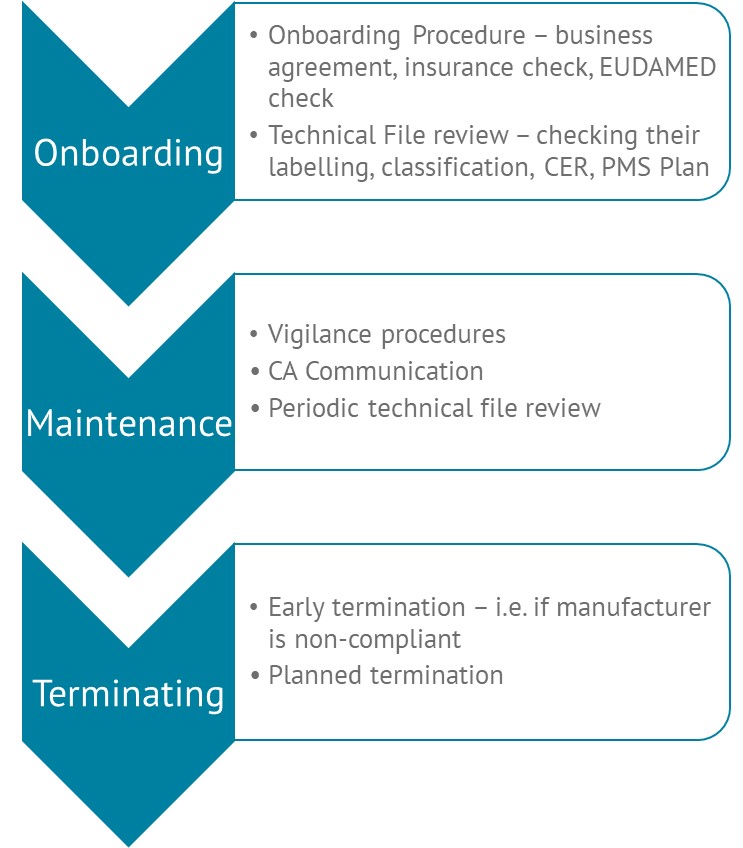
- Onboarding Process: We start with a thorough onboarding process where our team assesses the manufacturer’s regulatory, quality, legal, and technical documentation. This step is crucial to ensure compliance from the outset. Additionally, we assist with EUDAMED registration, a mandatory requirement under the MDR and IVDR.
- Documentation Management: Lumis takes on the responsibility of storing and archiving relevant technical and regulatory documentation. This service ensures that documents are readily accessible for the authorities whilst also protected under stringent security measures.
- EUDAMED Compliance: We verify the EUDAMED registration requirements are complied with and ensure ongoing compliance with this critical EU requirement.
- Regulatory Communication: Our team acts as the point of contact between the manufacturer and EU Competent Authorities. We manage communications, ensure that all regulatory updates are addressed, and respond to any queries from authorities on behalf of the manufacturer.
- Lifecycle Management: Throughout the product lifecycle, we review updated technical documentation as needed, ensuring that it remains compliant with evolving regulatory standards.
- Incident and Safety Reporting: We support manufacturers in meeting their obligations for reporting serious incidents, trend reports, periodic summary reports, and field safety corrective actions. This proactive approach helps mitigate risks and ensures timely compliance with EU regulations.
- Certificates of Free Sale: Lumis assists manufacturers in obtaining Certificates of Free Sale, which are essential for exporting medical devices to certain non-EU countries.
- Full Mandate Compliance: We perform all necessary activities as outlined in Article 11 (3) (a-h) of the MDR/IVDR, ensuring that the manufacturer’s obligations under the EU regulations are fully met.
A Dedicated Team for Seamless Compliance
Lumis prides itself on offering a seamless experience for manufacturers. From the initial onboarding to ongoing support, a dedicated Lumis team is assigned to each manufacturer. This team is responsible for verifying that all regulatory requirements are continually met, ensuring that the manufacturer can focus on their core business operations without worrying about compliance issues.
By partnering with Lumis, manufacturers can swiftly access the EU market with confidence, knowing they have a trusted partner to handle all aspects of Authorized Representation.
For more information about our Authorized Representation Services or to discuss your specific needs, please contact Lumis today – see below for contact details. We are committed to helping you achieve successful market entry and ongoing compliance in the European Union.
References
Regulation (EU) 2017/745 of the European Parliament and of the Council. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02017R0745-20240709
Regulation (EU) 2017/746 of the European Parliament and of the Council of 5 April 2017 on in vitro diagnostic medical devices. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02017R0746-20240709
MDCG 2022-16 – Guidance on Authorised Representatives Regulation (EU) 2017/745 and Regulation (EU) 2017/746. https://health.ec.europa.eu/document/download/0a7613cb-6b9a-4396-a4c6-d2479e43e167_en?filename=mdcg_202216_en.pdf

