The European Union’s Medical Device Regulation and the new Artificial Intelligence Act introduced a seismic shift for medical device companies. For developers of Software as a Medical Device (SaMD) and, specifically, Artificial Intelligence Medical Devices (AiMD), obtaining the coveted CE mark has become significantly more complex.
Category - Medical device regulatory consulting & services
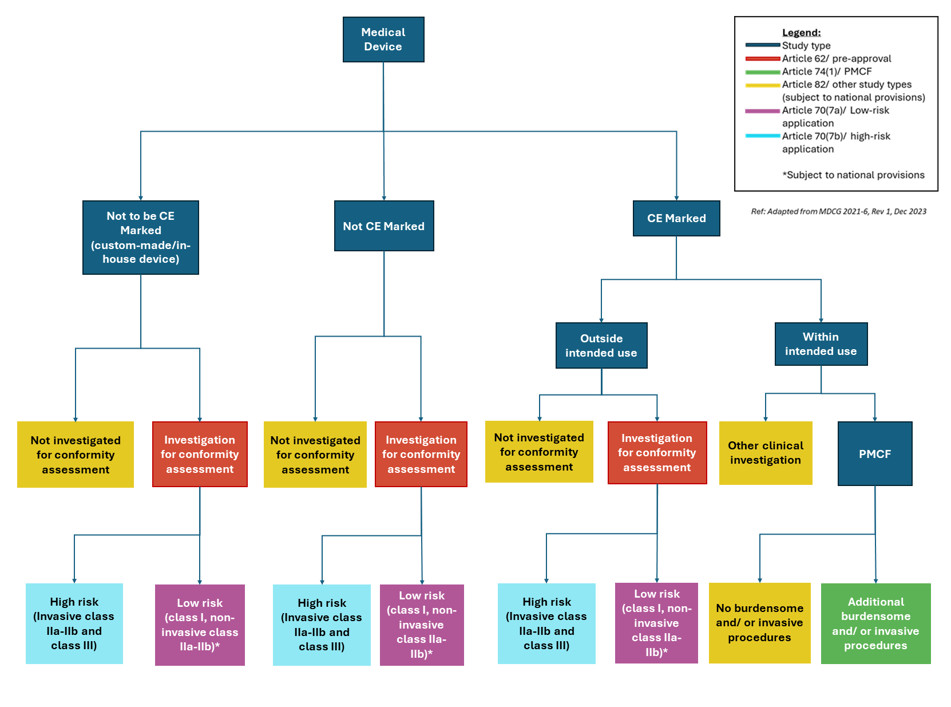
Regulatory Pathways for Medical Device Clinical Trials
This is a comprehensive review of the process, pitfalls, and procedures to get study approval for medical device clinical trials. We look into the future and unpack the present, in terms of European medical device regulation and the local regulatory landscape. Along with the intricacies of EU MDR 2017/745 medical device regulations, we provide an important summary of medical device study classifications and the corresponding submission pathways.