Lumis videos and webinars
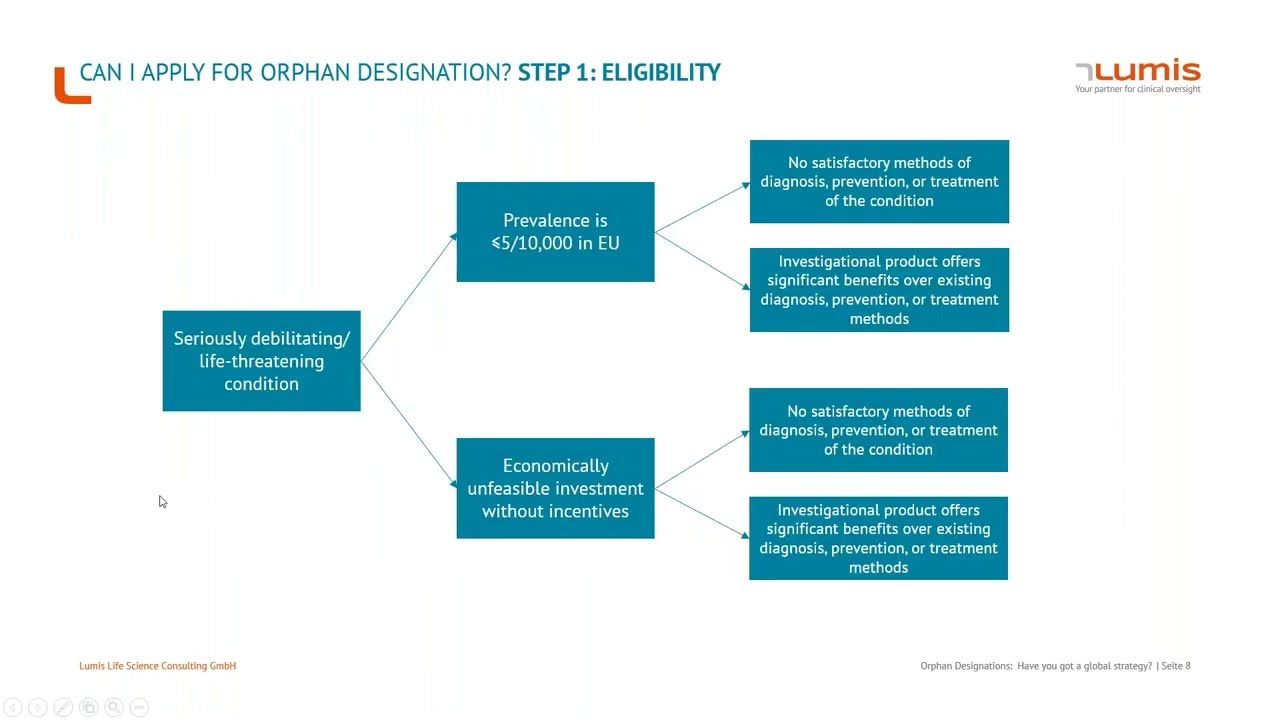
Webinar: How to devise a global orphan designation strategy
Developing a winning regulatory strategy for Orphan Designation across the EU, USA and APAC.
Webinar: How to devise a global orphan designation strategy Watch
Heike Schön, Lumis International GmbH Interview with BlueCloud TV
Heike Schön of Lumis International GmbH interviewed on BlueCloud TV
Heike Schön, Lumis International GmbH Interview with BlueCloud TV Watch
Webinar: Verify your clinical study design with regulators & manage your vendors efficiently
In this webinar we focus on the optimization of scientific clinical plans, clinical vendor management and contracting. With rapidly changing standards of care, the appropriate clinical study design can be a moving target.
Webinar: Verify your clinical study design with regulators & manage your vendors efficiently Watch
Webinar: Patients First! Decentralization – The (new) Clinical Trial Paradigm
In this webinar we explore decentralized clinical study concepts, their implications and challenges, how to simplify complicated workflows with eConsent and how stakeholders collaborate in decentralized clinical trials.
Webinar: Patients First! Decentralization – The (new) Clinical Trial Paradigm Watch
Webinar: Smart Sponsor, Small Budget? Smart Outsourcing
In this webinar we explain how smart outsourcing and smart contracting is your basis for selecting the best fitting CRO. You will gain the following insights: Best practices to select the most suitable CRO, create an effective outsourcing toolkit and successfully contracting with CROs.
Webinar: Smart Sponsor, Small Budget? Smart Outsourcing Watch
Webinar: KPIs – Your key to successful clinical trial oversight!
Implementing Key Performance Indicators (KPIs) is crucial for a successful clinical trial. A core, manageable set of KPIs helps you to predict and assess the achievement of your goals. KPIs allow for continuous assessment of the clinical trial indicate and highlights the need for any changes. In this webinar we introduce the concept of KPIs, how to develop KPIs, presenting case studies and introducing key quality indicators.
Webinar: KPIs – Your key to successful clinical trial oversight! Watch
Webinar: Effective Vendor Management
Effective vendor management is crucial for the smooth running of a clinical trial. In this webinar we share key tips on implementing an effective and efficient vendor management system. We guide you on how to best select appropriate CROs and other vendors, how to effectively contract and manage vendors, develop risk-based assessments, measure vendor performance and create a relationship fostered upon trust and communication.
Use of technologies in Decentralised Clinical Trials supporting participant-centric approach
Lumis presents the different technologies which are used in Decentralised Clinical Trials to support the participant-centric approach. For example: eConsent, the electronic clinical outcome assessment or telehealth visits. The measurements carried out by these technologies are now more often being used as the main measurement of which the clinical study trial is trying to assess. This is what makes these new technologies so exciting and challenging.
Use of technologies in Decentralised Clinical Trials supporting participant-centric approach Watch
Change Management – Sponsors to navigate the uncertainties
In this short video clip Heike Schön looks at what sponsors need to consider when navigating the changes and uncertainties of Decentralised Clinical Trials. It is crucial to understand and become familiar with the new technologies which are implemented for these new types of clinical studies.
Change Management – Sponsors to navigate the uncertainties Watch
Decentralised Clinical Trials – Recent Top Drivers and Challenges
Here we talk to you through some of the factors that have sparked the increase in the use and challenges of Decentralised clinical trials. Especially with the start of the COVID-19 pandemic, companies were faced with the challenge to keep the patients in their ongoing clinical trials. Regulatory authorities also had to adjust quickly to support the new approaches to clinical trials.
Decentralised Clinical Trials – Recent Top Drivers and Challenges Watch